So my colleague Kathleen and her inexhaustable supply of undergrads at Simon Fraser University in B.C., Canada, have generated seven potential DMAP1 lesions, using the male recombination screen described in the last post. Recall that in this screen, a P transposable element very close to the upstream gene CG33785 was induced to transpose in the male germline, hopefully generating a deletion in the direction of DMAP1, and thereby recombining a recessive brown eye colour mutation onto the same chromosome. Note here that four out of seven potential lesions produce viable brown-eyed (and therefore homozygous) flies. They look normal but are they really? In Figure 1, each potential mutant that produces homozygous progeny is tested for fertility. For each strain, four crosses, with virgin male and female parents, 3-4 of each sex (i.e, as close to identical conditions as possible). First, balanced males and females are crossed to each other (grey and black representing Cy males and females respectively – remember Cy is the dominant curly wing mutation that marks the SM6 balancer). Then, balanced males are crossed to homozygous females (which are dark blue in my colour scheme) – this tests the homozygous female fertility. Then the other way around: homozygous males (light blue) crossed to balanced females – this tests the homozygous males’ fertility. Finally, homozygous males and females to each other.
The total number of flies for each cross exceeded 100, but I expressed the proportions of each genotype as a percentage of 100 in this graph. For balanced parents crossed to each other, the ratio of balanced to non balanced progeny (or blue shades to grey shades) should be 0.5. (Think A/a X A/a, where A is Cy, and Cy/Cy is lethal. Half as many a/a to A/a, or 1/3 : 2/3). Note that doesn’t really happen except for 12-14 (and that ratio turns out to be 0.48). For the two middle crosses, heterozygous males to homozygous females and the other way around (think A/a X a/a) half the progeny should be homozygous, and half should be balanced, or 1:1. So you should see as much grey/black and light/dark blue for those crosses. Do you? Of course, homozygotes crossed to each other are depicted solely in the blue shades.
Clearly two of the potential mutants show sterility defects: 20-13 is male AND female sterile (no progeny at all for the second, third and fourth crosses), and 12-14 looks male sterile. Incidentally, in the very first post about this project, I yanked the ovaries out of 20-13/20-13 females, (look here), and asserted the testes didn’t look quite right either, so that’s consistent so far. (Could still be something in the background, but relish it for now!)
As for 20-11 and K2-13, the second and third crosses don’t produce quite the right ratios. In addition, 20-11/20-11 parents produced approximately 70% fewer progeny than 20-11/SM6 parents, and for K2-13 that reduction was around 50%. So there might be viability and fertility issues for both lines.
In summary, 20-13 is homozygous male and female sterile, 12-14 is male sterile, 20-11 and K2-13 homozygotes may be semi fertile and perhaps even semi viable (particularly 20-11). But are these all mutations in the same gene? Stay tuned.


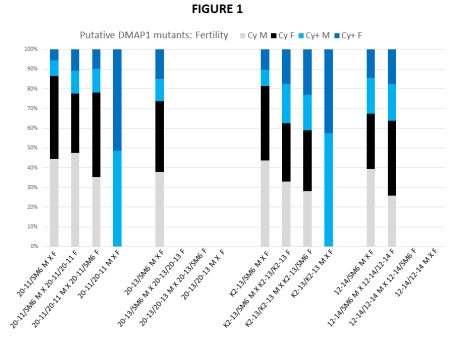

For the 20-11 crosses, I do recall that the 20-11 homozygotes are slower to develop than their heterozygous sibs. I wonder if the skew towards a greater proportion of curly winged flies might be explained that way.
LikeLike
Yes it could be? Certainly I scored the flies frequently and counted pupae that had not hatched, and tried to score all of the F1 progeny possible, at least in the first two vials. The uneclosed pupae that remained never hatched. But I guess if the development was slowed so that it overlapped considerably with the F2, we might not see that. But I estimated that 20-11/20-11 were delayed by about 24 hrs when compared with their het sibs (this at a lowish room temp of about 20 degrees celcius). By the way, did you say one of the lethals produced homozygous progeny under certain conditions?
LikeLike
I thought I saw some homozygotes dead in the bottom of the vials of 22-13-bw/SM6a when raised at 26C. It is hard to tell because the eyes darken once they are dead but I saw one brown eyed fly half out of the pupal case and it had just recently died. I tried to supplement the food with live yeast to see if I could get some of them to the adult stage but it didn’t work (last time I saw homozygotes in that stock was in early January of this year).
Kassandra has been working on lethal phase analysis, and she finds that the lethal phase is after mid-larval phase.
LikeLike
22-13 is one of the lethal lines. I rebalanced all of them over CyO-GFP and so far can find no GFP minus L3 larvae in 10-14, suggesting an earlier lethal phase. We should talk about this more in the next post, which will be about the interse crosses I did and the deficiency complementation you did.
LikeLike
Still referring to 22-13- bw there.
LikeLike
I wonder if we could restore the 22-13 delayed eclosion (but sterility of homozygous flies) by outcrossing. Not that I have time….but it would be interesting, I assume that the current homozygous lethality is due to accumulation of modifiers. If they are not on chromosome 2 it might just take a couple of generations to cross them out.
LikeLike